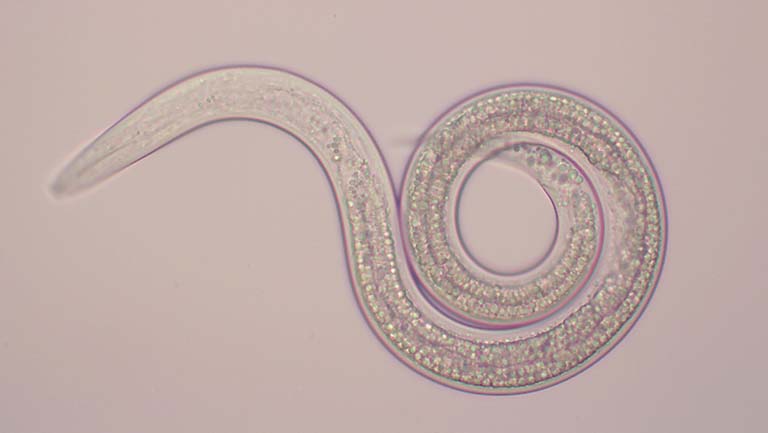
X. bovienii is both a specialized mutualistic symbiont of nematodes and a broadly virulent insect pathogen. The researchers found a strong signature of nematode host association as well as evidence for gene flow connecting isolates associated with different nematode host species. Further, they saw signatures of selection for genes involved with nematode host associations, insect pathogenicity, and microbial competition.
“We went into the project expecting that this bacterial species would be divided into different lineages based on their nematode host associations. While we did see a strong signal of these host associations, it wasn’t strict, suggesting that occasionally these microbes shift their nematode partners,” said Bashey-Visser. “Further, all the isolates that we sequenced were connected to each other by recent genetic exchange, regardless of whether they were found associated with a different nematode host.”
Although microbes reproduce clonally, they are notorious for acquiring genes from other microbial species. This is a major contributor to the spread of antibiotic resistance, as resistance genes can be transferred from one microbe to another when they are found in proximity.
“Often, we identify these gene transfer events by detecting a gene from a totally different species that stands out against the rest of the genome; in our study we identified genetic exchange by identifying regions of higher-than-expected similarity,” noted Bashey-Visser.
Where examined, microbe populations show frequent recombination, gaining and losing new gene fragments or whole sets of genes. Thus, a newly arising allele can spread in a microbial population, across clonal lineages, similarly to what is seen in sexually reproducing species.
The researchers found several genes associated with biotic interactions to be undergoing selective sweeps. In addition to genes important to interactions with the nematode host, insect toxins and genes implicated in microbial competition were also found to differentiate within the region. The newly sequenced isolates were all originally collected at IU Teaching and Research Preserve sites as part of National Science Foundation funded research led by Bashey-Visser and Curt Lively, a professor in the Department of Biology, who is also a co-author on the study. Douglas Rusch, lead bioinformatician at IU Center for Genomics and Bioinformatics, was a critical member of the team, designing the sequencing approach and key steps of the analysis. Much of the work of isolating the bacteria and identifying the nematode hosts was completed by undergraduates involved in IU’s Science, Technology, and Research Scholars; the Center of Excellence for Women & Technology; and Women in STEM Living-Learning Center or Biology X490 Independent Study programs. The immediate funding for the work was from the Office of the Vice Provost of Research at Indiana University‐Bloomington through the Collaborative Research and Creative Activity Funding Award.
Bashey-Visser and Papudeshi concluded that gene flow maintains cohesiveness across host associations in X. bovienii and may facilitate adaptive responses to a multi-partite selective environment.


 The College of Arts
The College of Arts