Abstract
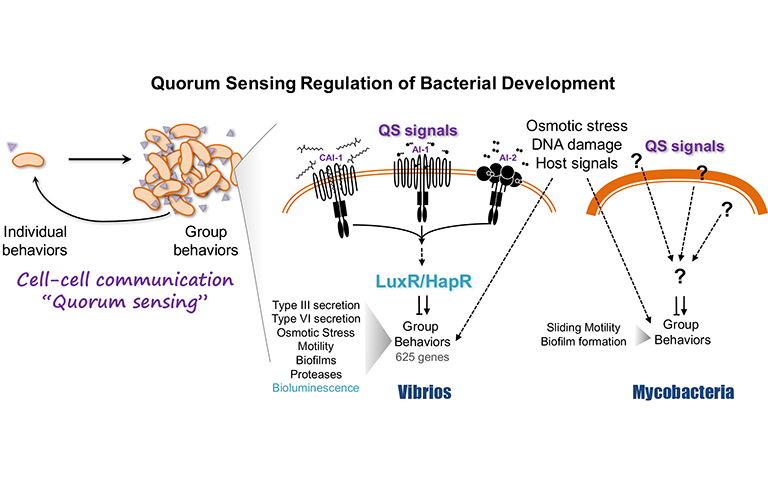
Bacteria communicate using the cell-cell signaling system called quorum sensing to collectively alter gene expression in response to changes in cell density. Quorum sensing controls behaviors that benefit the group for adaption and survival, including biofilm formation, motility, bioluminescence, and virulence factor secretion. Molecules that modulate quorum sensing have potential use as anti-microbial drugs aimed at bacteria that use quorum sensing to control virulence. Thus, there is a critical need to comprehensively understand how cell-cell signaling regulates virulence and impacts bacteria in their environmental niches.
Despite advances in elucidating the quorum signaling inputs, comparatively less is known about the output – the transcriptional regulation program that controls group behaviors. The objective of my research is to define how bacteria use quorum sensing signaling to control virulence gene expression. Toward this goal, we study quorum sensing gene regulation in Vibrios, both as relevant pathogens and as established quorum sensing model systems. Quorum sensing was first elucidated in bioluminescent Vibrios, and these bacterial systems now serve as exceptional models for the quorum sensing pathways that are mirrored in the human pathogens Vibrio cholerae, Vibrio vulnificus, and Vibrio parahaemolyticus. In Vibrios, virulence gene expression is controlled by the LuxR-type master transcription factors at the center of the quorum sensing pathway. My work has shown that quorum sensing and LuxR activate and repress hundreds of genes in response to quorum signals that impinge on cell growth and physiology.
My lab will expand this foundation over the next five years to establish links between quorum sensing and gene expression. We have uncovered novel mechanisms of gene regulation by LuxR in concert with nucleoid-associated proteins that are critical for precise timing and expression levels of quorum sensing genes. My lab will extend this work by determining the regulatory mechanism of the central LuxR/HapR proteins in pathogenic Vibrios, which are key targets for developing inhibitors of quorum sensing. Further, we have shown that the quorum sensing pathway intersects other signaling pathways (e.g., the osmotic stress response pathway). We will use temporal, singlecell, and population-level experiments to study how gene expression is coordinated in response to changes in both cell density and the local environment during growth and host colonization. Finally, we are now extending our knowledge and methodological approaches to examine quorum sensing gene regulation in the Mycobacteria, which includes the prevalent human pathogen Mycobacterium tuberculosis. We have initiated studies to identify the quorum sensing gene network in Mycobacteria and determine its impact on virulence phenotypes.
Overall, this work will provide the fundamental data critical to understanding quorum signaling and how it impacts bacterial pathogenesis to contribute to future advances in disease treatment.


 The College of Arts
The College of Arts